What is Burgess Reagent?
CAS: 29684-56-8 | Eburon Organics ref.: 100.110
(Methoxycarbonylsulfamoyl)-triethylammonium hydroxide inner salt is called after Edward Meredith Burgess (The Georgia Institute of Technology, Atlanta, Georgia USA). The Burgess reagent is a carbamate and used for the dehydration of various sec- and tert- alcohols to get the corresponding olefins. Moreover, it also found applications in numerous synthetic transformations in organic chemistry and particularly in medicinal chemistry.
Applications of Burgess Reagent in Synthetic organic Chemistry
More than just a powerful dehydrating agent for alcohols, it can smoothly react with several
nucleophilic functional groups such as:
- Alcohols
- Epoxides
- 1.2-diols
- Thiols
- Oximes
- ...
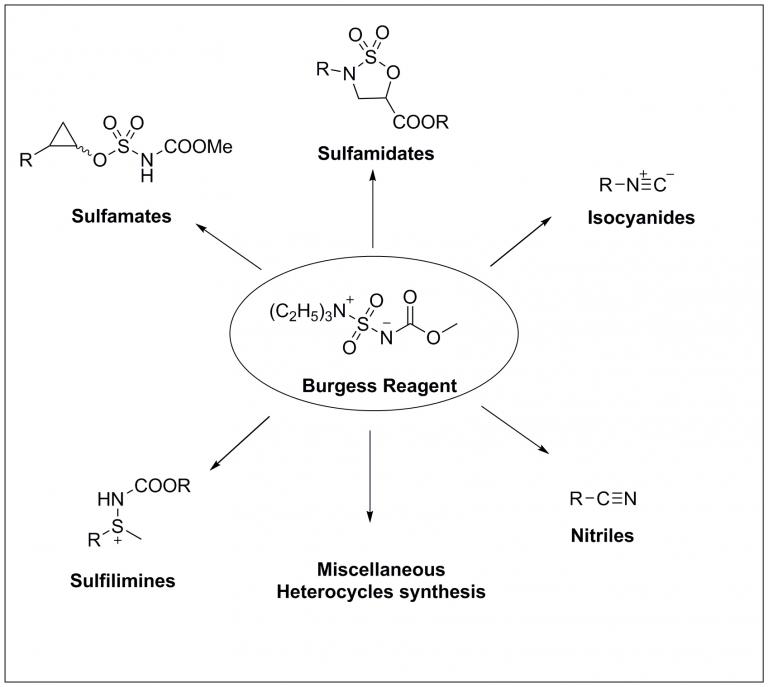
Significantly, it has also been used in the synthesis of:
- Sulfamidates
- Sulfamates
- Sulfilimines
- ...
Further, synthesis of biologically active natural products is efficiently promoted by Burgess reagent.